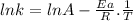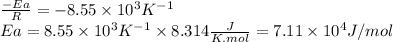The rate constant of a reaction is measured at different temperatures. a plot of the natural log of the rate constant as a function of the inverse of the temperature (in kelvins) yields a straight line with a slope of −8.55×103 k−1. what is the activation energy (ea) for the reaction?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

You know the right answer?
The rate constant of a reaction is measured at different temperatures. a plot of the natural log of...
Questions

Advanced Placement (AP), 26.07.2019 14:30

Advanced Placement (AP), 26.07.2019 14:30


Advanced Placement (AP), 26.07.2019 14:30

Advanced Placement (AP), 26.07.2019 14:30

Advanced Placement (AP), 26.07.2019 14:30

Advanced Placement (AP), 26.07.2019 14:30

Physics, 26.07.2019 14:30

English, 26.07.2019 14:30


Advanced Placement (AP), 26.07.2019 14:30



Social Studies, 26.07.2019 14:30




English, 26.07.2019 14:30

Mathematics, 26.07.2019 14:30

Mathematics, 26.07.2019 14:30