
Water’s specific heat capacity is 4.186 joules/gram degree celsius. mercury’s specific heat capacity is 0.140 joules/gram degree celsius.
water and mercury are put into three identical bowls:
bowl a contains 20 grams of water.
bowl b contains 40 grams of water.
bowl c contains 20 grams of mercury.
the bowls start at the same temperature, and then the same amount of heat is added to each bowl. order the bowls from coolest to warmest, based on their final temperatures.
bowl a

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:30
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2
You know the right answer?
Water’s specific heat capacity is 4.186 joules/gram degree celsius. mercury’s specific heat capacity...
Questions

Mathematics, 29.04.2021 22:30



Mathematics, 29.04.2021 22:30


Mathematics, 29.04.2021 22:30

History, 29.04.2021 22:30






Mathematics, 29.04.2021 22:30






Advanced Placement (AP), 29.04.2021 22:30

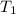
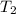
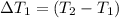
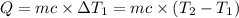
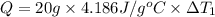
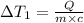
 ..[1]
..[1]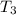

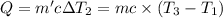
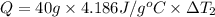
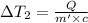
 ..[2]
..[2]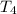
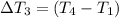

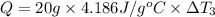
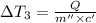
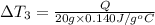 ..[3]
..[3]



