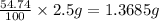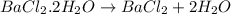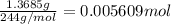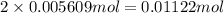Chemistry, 19.11.2019 01:31 sixtomomtermont
Astudent had a mixture of sand and barium chloride dihydrate (bacl2x2h2o). a beaker containing the mixture weighed 72.248g. when empty the beaker weighed 69.748g. the mixture was determined to contain 45.26% sand.
(a) what is the % and mass of the hydrate in the mixture?
(b) if the mixture was selectively decomposed by heating, how many grams and moles of water would be lost?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1


Chemistry, 23.06.2019 12:10
What is the correct name for hg(no3)2? mercury (i) nitrate mercury (ii) nitrate mercury nitroxide mercury dinitride
Answers: 1
You know the right answer?
Astudent had a mixture of sand and barium chloride dihydrate (bacl2x2h2o). a beaker containing the m...
Questions



Mathematics, 10.02.2021 03:00

English, 10.02.2021 03:00



Mathematics, 10.02.2021 03:00

Mathematics, 10.02.2021 03:00

Mathematics, 10.02.2021 03:00


English, 10.02.2021 03:00


Biology, 10.02.2021 03:00

History, 10.02.2021 03:00


Mathematics, 10.02.2021 03:00

Chemistry, 10.02.2021 03:00


History, 10.02.2021 03:00

English, 10.02.2021 03:00