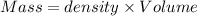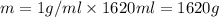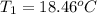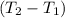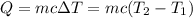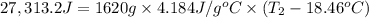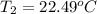Aquantity of 8.10 × 102 ml of 0.600 m hno3 is mixed with 8.10 × 102 ml of 0.300 m ba(oh)2 in a constant-pressure calorimeter of negligible heat capacity. the initial temperature of both solutions is the same at 18.46°c. the heat of neutralization when 1.00 mol of hno3 reacts with 0.500 mol ba(oh)2 is −56.2 kj/mol. assume that the densities and specific heats of the solution are the same as for water (1.00 g/ml and 4.184 j/g · °c, respectively). what is the final temperature of the solution?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2


Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1
You know the right answer?
Aquantity of 8.10 × 102 ml of 0.600 m hno3 is mixed with 8.10 × 102 ml of 0.300 m ba(oh)2 in a const...
Questions

Chemistry, 24.11.2021 17:30

English, 24.11.2021 17:30

English, 24.11.2021 17:30

Computers and Technology, 24.11.2021 17:30







Mathematics, 24.11.2021 17:30

Spanish, 24.11.2021 17:30

Social Studies, 24.11.2021 17:30


History, 24.11.2021 17:30


Arts, 24.11.2021 17:40



English, 24.11.2021 17:40

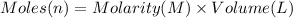
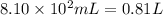
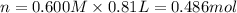
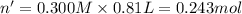
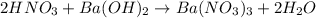
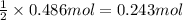 barium hydroxide.
barium hydroxide.