Define the term ionization energy. choose one:
a. the amount of energy needed to remove 1 mole of electrons from 1 mole of ground-state atoms or ions in the gas phase.
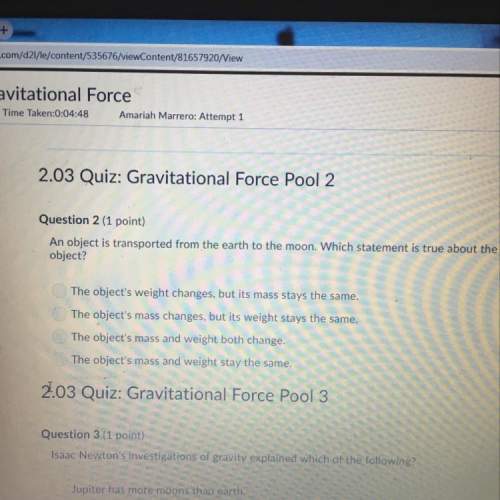
b. a positively charged particle created when an atom or molecule loses one or more electrons.
c. a relative measure of the ability of an atom to attract electrons in a bond to itself.
d. the amount of energy created by the positive or negative forces of ions.
e. the energy change that occurs when 1 mole of electrons combines with 1 mole of atoms or ions in the gas phase.
f. the energy released when an electron is attracted to a nucleus, thus fulfilling a valence shell octet and creating internal atomic homeostasis

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:50
How do the energy differences between the higher energy levels of an atom compare with the energy difference between the lower energy level of the atom
Answers: 1

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 13:10
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes.which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature.sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
Define the term ionization energy. choose one:
a. the amount of energy needed to remove 1 mo...
a. the amount of energy needed to remove 1 mo...
Questions


Mathematics, 27.05.2020 17:57

Mathematics, 27.05.2020 17:57





Geography, 27.05.2020 17:57

Mathematics, 27.05.2020 17:57





Mathematics, 27.05.2020 17:57





Mathematics, 27.05.2020 17:57