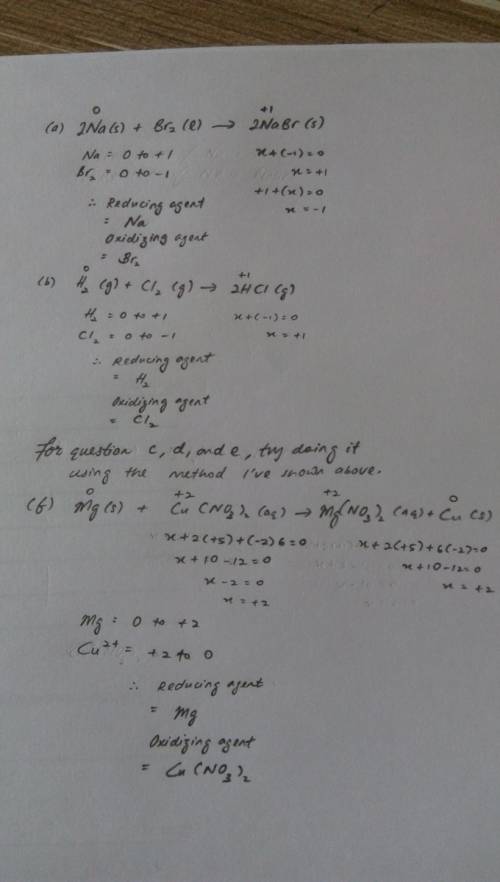Use electron transfer or electron shift to identify what is oxidized and what is reduced in each reaction :
a) 2na(s) + br2(l) > 2nabr(s)
b) h2(g) + cl2(g) > 2hcl(g)
c) 2li(s) + f2(g) > 2lif(s)
d) s(s) + cl2(g) > scl2(g)
e)n2(g) + 2o2(g) > 2no2(g)
f) mg(s) +cu(no3)2(aq) = mg(no3)2(aq) + cu(s)
for each reaction above, identify the reducing agent and the oxidizing agent

Answers: 1
Another question on Chemistry

Chemistry, 20.06.2019 18:04
Acompound is found to contain 6.1% hydrogen and 93.9% oxygen. find it’s empirical formula.
Answers: 2

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
Use electron transfer or electron shift to identify what is oxidized and what is reduced in each rea...
Questions



Physics, 29.04.2021 01:50


Spanish, 29.04.2021 01:50


History, 29.04.2021 01:50

Social Studies, 29.04.2021 01:50

Mathematics, 29.04.2021 01:50

Mathematics, 29.04.2021 01:50


Mathematics, 29.04.2021 01:50

Mathematics, 29.04.2021 01:50

History, 29.04.2021 01:50



Mathematics, 29.04.2021 01:50