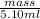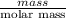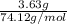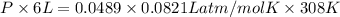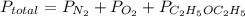Asample of 5.10 ml of diethylether (c2h5oc2h5; density = 0.7134 g/ml) is introduced into a 6.00 -l vessel that already contains a mixture of n2 and o2, whose partial pressures are pn2 = 0.752 atm and po2 = 0.206 atm. the temperature is held at 35.0 °c, and the diethylether totally evaporates.
a) calculate the partial pressure of the diethylether.
b) calculate the total pressure in the container.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

You know the right answer?
Asample of 5.10 ml of diethylether (c2h5oc2h5; density = 0.7134 g/ml) is introduced into a 6.00 -l...
Questions





History, 30.01.2020 12:48

Biology, 30.01.2020 12:48

Mathematics, 30.01.2020 12:48





Geography, 30.01.2020 12:48

Arts, 30.01.2020 12:48

Health, 30.01.2020 12:48


Social Studies, 30.01.2020 12:48


Mathematics, 30.01.2020 12:48


History, 30.01.2020 12:48

 = (35 + 273) K = 308 K
= (35 + 273) K = 308 K