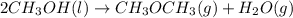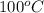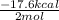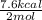Given the standard heat of combustion of methanol ch3oh is 182.6
kcal/mol, dimethyl ether ch3och3 is 347.6 kcal/mol, (methanol/
dimethyl ether in gas phase, water in liquid phase). given the heat
of vaporization of water is 10 kcal/mol, methanol is 8.4 kcal/mol,
dimethyl ether 4.8 kcal/mol. calculate the reaction of dehydration
of methanol to produce dimethyl ether. (indicate the phase of your
components).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
Given the standard heat of combustion of methanol ch3oh is 182.6
kcal/mol, dimethyl ethe...
kcal/mol, dimethyl ethe...
Questions

English, 11.10.2021 01:00



History, 11.10.2021 01:00



Mathematics, 11.10.2021 01:00


Mathematics, 11.10.2021 01:00

English, 11.10.2021 01:00

English, 11.10.2021 01:00


Mathematics, 11.10.2021 01:00


Biology, 11.10.2021 01:00

Engineering, 11.10.2021 01:00



Mathematics, 11.10.2021 01:00

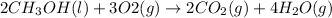 ....... (1)
....... (1)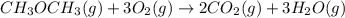 -347.6 kcal/mol
-347.6 kcal/mol
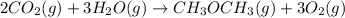 heat of reaction +347.6 kcal {sign reversed with reaction} ........ (2)
heat of reaction +347.6 kcal {sign reversed with reaction} ........ (2)