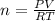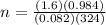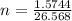Chemistry, 12.11.2019 04:31 ChloeLiz7111
Consider the chemical reaction:
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required to form 1.6 l of o2 at a temperature of 324 k and a pressure of 0.984 atm ?
express your answer using two significant figures.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
Consider the chemical reaction:
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required...
2h2o(l)→2h2(g)+o2(g)
how many moles of h2o are required...
Questions


Mathematics, 05.05.2020 11:00


Mathematics, 05.05.2020 11:00

Mathematics, 05.05.2020 11:00



English, 05.05.2020 11:00

Social Studies, 05.05.2020 11:00



Mathematics, 05.05.2020 11:00




Mathematics, 05.05.2020 11:00

Arts, 05.05.2020 11:00



Physics, 05.05.2020 11:00