Use the chart to determine which pair of atoms has the greatest polarity
...

Chemistry, 12.11.2019 03:31 venancialee8805
Use the chart to determine which pair of atoms has the greatest polarity
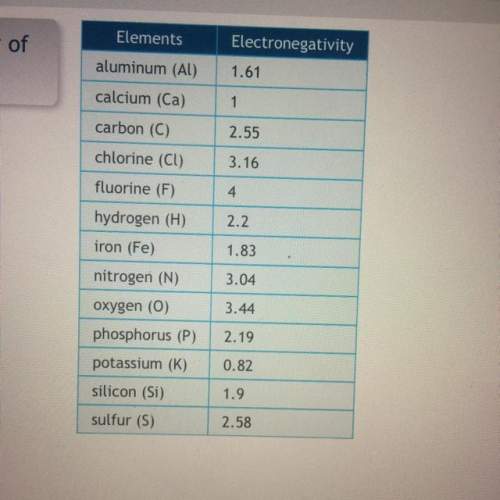

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Problem page combustion of hydrocarbons such as pentane ( c5 h12 ) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earth's atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide.(a) write a balanced chemical equation, including physical state symbols, for the combustion of liquid pentane into gaseous carbon dioxide and gaseous water. (b) suppose 0.350 kg of pentane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 degree c. calculate the volume of carbon dioxide gas that is produced.be sure your answer has the correct number of significant digits.
Answers: 2

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3


Chemistry, 23.06.2019 19:40
The reaction: (ch3)3cbr + oh− → (ch3)3coh + br− in a certain solvent is first order with respect to oh−. in several experiments, the rate constant k was determined at different temperatures. a plot of ln(k) vs 1/t was constructed and the slope of the line was -1.10 x 104 k with a y-intercept of 33.5. what is the value for k at a temperature of 25°c?
Answers: 1
You know the right answer?
Questions

Mathematics, 27.02.2021 08:20


Mathematics, 27.02.2021 08:20



Mathematics, 27.02.2021 08:20

Chemistry, 27.02.2021 08:20


Chemistry, 27.02.2021 08:20


Mathematics, 27.02.2021 08:20

Chemistry, 27.02.2021 08:20

Chemistry, 27.02.2021 08:20

English, 27.02.2021 08:20


Mathematics, 27.02.2021 08:20

Mathematics, 27.02.2021 08:20

English, 27.02.2021 08:20


English, 27.02.2021 08:20



