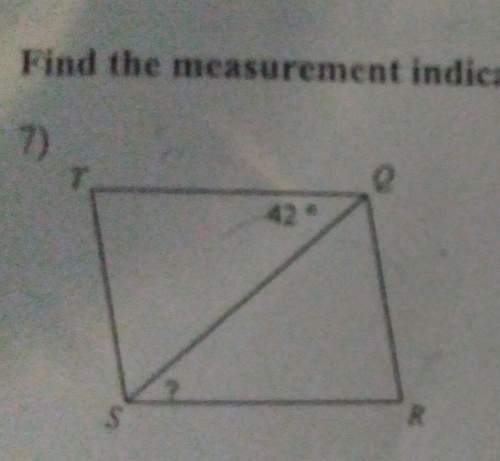
This is find the measurment im confused about it
...

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:40
Experiment: effect of solution concentration on reaction rate you have learned that as the concentration of reactants increases, there will most likely be a greater number of collisions, and hence increase the rate of a reaction. in this experiment, you will see a demonstration of this, with a twist. there will be three reactions going on in this experiment. objectives determine how solution concentration can affect the rate of a reaction. the first reaction will be a reaction of the iodide ion (i-1) with hydrogen peroxide (h2o2) in an acidic solution. this reaction produces a slightly orange solution. in our experiment, we will add some orange food coloring to make this solution more orange. 2 h+ (aq) + 2 i- (aq) + h2o2 (aq) ⟶ i2 (aq) + 2 h2o (l) the next reaction will be between the iodine and starch i2 + starch ⟶ i2-starch complex (blue-black) so, when starch is added to the iodine solution made from the first reaction, the solution will turn black immediately, so it is difficult to find the rate of reaction. in order to be able to time this reaction, you will slow it down with another reaction. adding ascorbic acid will react with the iodine, reducing the concentration of the iodine available to react with the starch. c6h8o6 (aq) + i2 (aq) ⟶ 2i- (aq) + c6h6o6 (aq) + 2 h+ (aq) when the ascorbic acid is used up, the remaining iodine molecules can react with the starch and form the black color. the more ascorbic acid you add, the slower the reaction to form the iodine-starch complex will be. use your data and observations to complete the assignment. analysis and conclusions submit your data and the answers to these questions in the essay box below. what was your hypothesis? plot your data as drops of ascorbic acid vs. time. as the concentration of ascorbic acid was increased, did the rate of the formation of the iodine-starch complex increase or decrease? explain your answer in terms of the chemical reactions involved. was your hypothesis correct? make a general rule about the effects of concentration of reactants on reaction rates. for practice, the molecular formula for ascorbic acid is c6h8o6, and you used 6 g in this experiment, calculate the molarity of the ascorbic acid. now calculate the concentration in moles per drop (assume 1 ml = 20 drops).
Answers: 3

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

You know the right answer?
Questions

History, 22.02.2021 22:00


Mathematics, 22.02.2021 22:00

Mathematics, 22.02.2021 22:00

Social Studies, 22.02.2021 22:00

Mathematics, 22.02.2021 22:00



Mathematics, 22.02.2021 22:00


Law, 22.02.2021 22:00


Mathematics, 22.02.2021 22:00

Spanish, 22.02.2021 22:00

History, 22.02.2021 22:00



Spanish, 22.02.2021 22:00