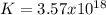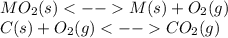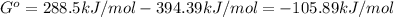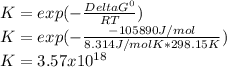Chemistry, 10.11.2019 07:31 lovelyheart5337
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s) < > m(s)+o2(g) delta g = 288.5 kj/mol
1.) when the reaction is coupled to the conversion of graphite to carbon dioxide, it becomes spontaneous. what is the chemical equation of this coupled process? show that the reaction is in equilibrium, include physical states, and represent graphite as c(s)
2.) what is the thermodynamic equilibrium constant for the coupled reaction? k =

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 07:30
Which statement explains which thermometer is more appropriate to measure the temperature of a liquid at 43.6 degrees celsius a) thermometer a, because it measures temperature more accurately than thermometer b b) thermometer b, because it measures temperature more accurately than thermometer a c) thermometer a, because it measures temperature more precisely than thermometer b d) thermometer b, because it measures temperature more precisely than thermometer a
Answers: 2

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1

Chemistry, 23.06.2019 10:00
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1

Chemistry, 23.06.2019 16:50
Question 2 (5 points) which of the following materials is useful for making molds because it has a low melting point? question 2 options: wood metal wax sand
Answers: 2
You know the right answer?
When the oxide of generic metal m is heated at 25c, only a negligible amount of m is produced. mo2(s...
Questions

English, 21.10.2019 13:30



Mathematics, 21.10.2019 13:30


Mathematics, 21.10.2019 13:30

Mathematics, 21.10.2019 13:30


Mathematics, 21.10.2019 13:30


Mathematics, 21.10.2019 13:30

Mathematics, 21.10.2019 13:30

English, 21.10.2019 13:30


Mathematics, 21.10.2019 13:30

Mathematics, 21.10.2019 13:30



Mathematics, 21.10.2019 13:30

Mathematics, 21.10.2019 13:30