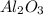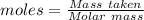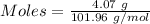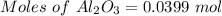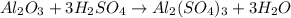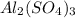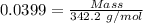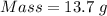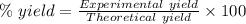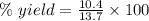Chemistry, 10.11.2019 03:31 babyboogrocks5572
For the following reaction, 4.07 grams of aluminum oxide are mixed with excess sulfuric acid. the reaction yields 10.4 grams of aluminum sulfate. aluminum oxide (s) + sulfuric acid (aq) aluminum sulfate (aq) + water (l) what is the theoretical yield of aluminum sulfate? grams what is the percent yield for this reaction? %

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
For the following reaction, 4.07 grams of aluminum oxide are mixed with excess sulfuric acid. the re...
Questions



Mathematics, 18.02.2021 14:00



Mathematics, 18.02.2021 14:00

Computers and Technology, 18.02.2021 14:00

Arts, 18.02.2021 14:00



Spanish, 18.02.2021 14:00

Physics, 18.02.2021 14:00

Mathematics, 18.02.2021 14:00



Mathematics, 18.02.2021 14:00

English, 18.02.2021 14:00

Biology, 18.02.2021 14:00


History, 18.02.2021 14:00