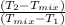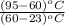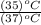Chemistry, 08.11.2019 06:31 JadeCaldwell
18. how many milliliters of water at 23 °c with a density of 1.00 g/ml must be mixed with 180 ml (about 6 oz) of coffee at 95 °c so that the resulting combination will have a temperature of 60 °c? assume that coffee and water have the same density and the same specific heat.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
18. how many milliliters of water at 23 °c with a density of 1.00 g/ml must be mixed with 180 ml (ab...
Questions



World Languages, 23.06.2021 18:10



Mathematics, 23.06.2021 18:10

Mathematics, 23.06.2021 18:10


Social Studies, 23.06.2021 18:10





Biology, 23.06.2021 18:10



Mathematics, 23.06.2021 18:10


Mathematics, 23.06.2021 18:10

Mathematics, 23.06.2021 18:10

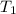 ) =
) = 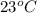
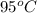
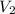 ) = 180 mL
) = 180 mL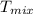 ) =
) = 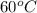
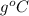 )
)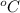 )
)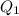 ) should be the same as the heat of coffee (
) should be the same as the heat of coffee (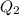 ). Thus,
). Thus,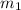 x
x 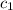 x Δ
x Δ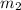 x
x 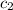 x Δ
x Δ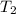
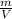
 x V
x V x V
x V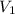 is the volume of water
is the volume of water