
Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo3(s)2kcl(s) + 3o2(g) the product gas, o2, is collected over water at a temperature of 25 °c and a pressure of 749 mm hg. if the wet o2 gas formed occupies a volume of 5.76 l, the number of moles of kclo3 reacted was mol. the vapor pressure of water is 23.8 mm hg at 25 °c.

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2
You know the right answer?
Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo...
Questions




History, 25.01.2020 14:31

Mathematics, 25.01.2020 14:31

Business, 25.01.2020 14:31


History, 25.01.2020 14:31

Geography, 25.01.2020 14:31


Chemistry, 25.01.2020 14:31

Mathematics, 25.01.2020 14:31



History, 25.01.2020 14:31





Mathematics, 25.01.2020 14:31

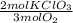 = 0,15 mol of KClO₃
= 0,15 mol of KClO₃

