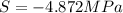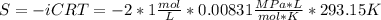Chemistry, 29.10.2019 21:31 tynyiaawrightt
Assuming that the nacl is completely ionized, calculate how much it will lower the solute potential of the soil at 20°c using the solute potential equation: ѱs = –icrt where i is the ionization constant (2 for nacl), c is the molar concentration (in mol/l), r is the pressure constant [r = 0.00831 l • mpa/(mol • k)], and t is the temperature in kelvin (273 + °c). how much will the solute potential of the soil be lowered at 20°c?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
Assuming that the nacl is completely ionized, calculate how much it will lower the solute potential...
Questions


Social Studies, 25.04.2020 01:33











Biology, 25.04.2020 01:34




Mathematics, 25.04.2020 01:34

Mathematics, 25.04.2020 01:34