Chemistry, 26.10.2019 03:43 Deavionaaaaa
An atom in its ground state is excited when it absorbs a single photon of light. the atom then relaxes back to the ground state by emitting two photons, the first, a red photon at 700 nm, and the second, an infrared photon at 1750 nm. what is the wavelength of the absorbed photon? 500 nm 1225 nm 700 nm 1950 nm 1750 nm

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
An atom in its ground state is excited when it absorbs a single photon of light. the atom then relax...
Questions

Mathematics, 14.07.2019 12:00




Biology, 14.07.2019 12:00

Biology, 14.07.2019 12:00






Chemistry, 14.07.2019 12:00







History, 14.07.2019 12:00

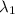 ) = 700 nm
) = 700 nm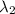 ) = 1750 nm
) = 1750 nm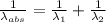
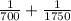
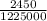
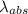 =
=