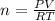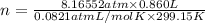Chemistry, 25.10.2019 19:43 nschavez123
Olympic cyclists fill their tires with helium to make them lighter. assume that the volume of the tire is 860 ml , that it is filled to a total pressure of 120 psi , and that the temperature is 26 ∘c. also, assume an average molar mass for air of 28.8 g/mol.
a) calculate the mass of air in an air filled tire.
b) calculate the mas of helium in a helium-filled tire.
c) what is the mass difference between the two?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
Olympic cyclists fill their tires with helium to make them lighter. assume that the volume of the ti...
Questions

Health, 13.03.2020 00:58

Mathematics, 13.03.2020 00:58

English, 13.03.2020 00:58








Business, 13.03.2020 00:58

Mathematics, 13.03.2020 00:58


Computers and Technology, 13.03.2020 00:58




Health, 13.03.2020 00:59