
Chemistry, 24.10.2019 17:43 odetteerivera
In the process for the manufacture of chlorine by direct oxidation of hcl with air over a catalyst to form cl2 and h2o (only), the exit product is composed of mole fractions of cl2 (21.4%), h2o (21.4%), and n2 (45.0%) – the remainder is hcl and o2. calculate and /or identify: (a) the limiting reactant? (b) the percentage excess reactant? (c) the degree of completion of the reaction? (d) the extent of reaction?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
In the process for the manufacture of chlorine by direct oxidation of hcl with air over a catalyst t...
Questions



Mathematics, 05.11.2021 01:40



Computers and Technology, 05.11.2021 01:40

Health, 05.11.2021 01:40

Computers and Technology, 05.11.2021 01:50





Biology, 05.11.2021 01:50

Mathematics, 05.11.2021 01:50

English, 05.11.2021 01:50



Mathematics, 05.11.2021 01:50


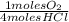 = 0,10725 moles of O₂
= 0,10725 moles of O₂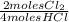 = 0,2145 moles of Cl₂ that are the same than moles of H₂O.
= 0,2145 moles of Cl₂ that are the same than moles of H₂O.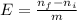
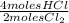 = 0,001 moles of HCl. As initial moles are 0,429 and stoichiometric coefficient is 4, extent of reaction is 0,107
= 0,001 moles of HCl. As initial moles are 0,429 and stoichiometric coefficient is 4, extent of reaction is 0,107

