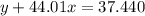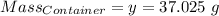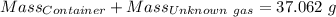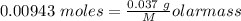Chemistry, 23.10.2019 20:30 camirialchambers17
Agas cylinder filled with nitrogen at standard temperature and pressure has a mass of 37.289 g. the same container filled with carbon dioxide at stp has a mass of 37.440 g. when filled with an unknown gas at stp, the container mass is 37.062 g. calculate the molecular weight of the unknown gas, and then state its probable identity.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
Agas cylinder filled with nitrogen at standard temperature and pressure has a mass of 37.289 g. the...
Questions


Physics, 22.07.2021 14:20

Chemistry, 22.07.2021 14:20


Social Studies, 22.07.2021 14:20

English, 22.07.2021 14:20

English, 22.07.2021 14:20

English, 22.07.2021 14:20

Mathematics, 22.07.2021 14:20

Geography, 22.07.2021 14:20


Mathematics, 22.07.2021 14:20


History, 22.07.2021 14:20

English, 22.07.2021 14:20

English, 22.07.2021 14:30




Physics, 22.07.2021 14:30

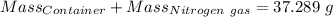
 = 28.014 g/mol
= 28.014 g/mol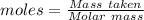

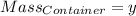
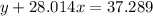

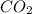 = 44.01 g/mol
= 44.01 g/mol