A2.52-g sample of a compound containing only carbon, hydrogen, nitrogen, oxygen, and sulfur was burned in excess oxygen to yield 4.23 g of co2 and 1.01 g of h2o. another sample of the same compound, of mass 4.14 g, yielded 2.11 g of so3. a third sample, of mass 5.66 g, was burned under different conditions to yield 2.27 g of hno3 as the only nitrogen containing product. determine the empirical formula of the compound.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
A2.52-g sample of a compound containing only carbon, hydrogen, nitrogen, oxygen, and sulfur was burn...
Questions

Mathematics, 07.10.2019 00:00



Mathematics, 07.10.2019 00:00

English, 07.10.2019 00:00

Mathematics, 07.10.2019 00:00

Spanish, 07.10.2019 00:00

Mathematics, 07.10.2019 00:00

Mathematics, 07.10.2019 00:00






Health, 07.10.2019 00:00

Mathematics, 07.10.2019 00:00




Arts, 07.10.2019 00:00

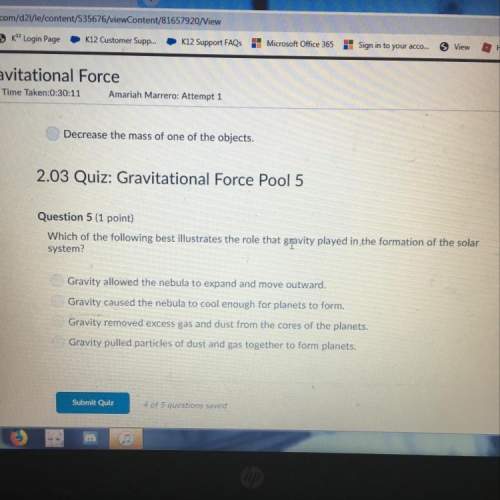
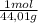 = 0,096 moles of CO₂ ≡ moles of C×12,01 g/mol = 1,15296 g of C/ 2,52*100 = 45,80 wt%
= 0,096 moles of CO₂ ≡ moles of C×12,01 g/mol = 1,15296 g of C/ 2,52*100 = 45,80 wt% 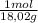 = 0,056 moles of H₂O = 0,112 moles of H×1,01 g/mol= 0,1132 g of H/2,52*100 = 4,49 wt%
= 0,056 moles of H₂O = 0,112 moles of H×1,01 g/mol= 0,1132 g of H/2,52*100 = 4,49 wt% = 0,026 moles of SO₃ = 0,026 moles of S×32,065 g/mol= 0,8457 g of S/4,14*100 = 20,43 wt%
= 0,026 moles of SO₃ = 0,026 moles of S×32,065 g/mol= 0,8457 g of S/4,14*100 = 20,43 wt%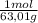 = 0,036 moles of H₂O = 0,036 moles of N×14 g/mol= 0,504g of H/5,56*100 = 9,07 wt%
= 0,036 moles of H₂O = 0,036 moles of N×14 g/mol= 0,504g of H/5,56*100 = 9,07 wt%