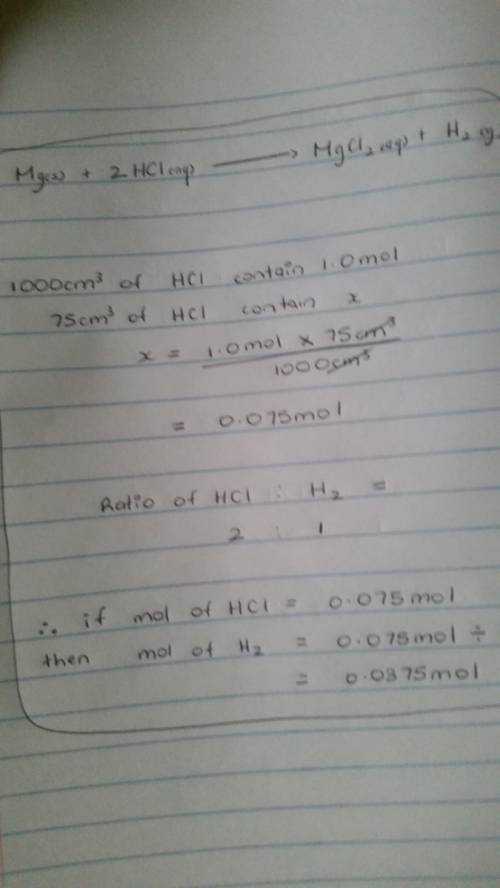Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
You know the right answer?
In the reaction mg (s) + 2hcl (aq) h2 (g) + mgcl2 (aq), how many moles of hydrogen gas will be produ...
Questions

Biology, 20.08.2019 04:30

Geography, 20.08.2019 04:30


History, 20.08.2019 04:30

English, 20.08.2019 04:30

Chemistry, 20.08.2019 04:30

Mathematics, 20.08.2019 04:30

Mathematics, 20.08.2019 04:30

Mathematics, 20.08.2019 04:30



Physics, 20.08.2019 04:30


Social Studies, 20.08.2019 04:30


Mathematics, 20.08.2019 04:30

Physics, 20.08.2019 04:30

Mathematics, 20.08.2019 04:30