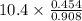Chemistry, 18.10.2019 19:10 Chrissyx4750
Suppose the reaction between nitrogen and hydrogen was run according to the amounts presented in part a, and the temperature and volume were constant at values of 303 k and 2.00 l, respectively. if the pressure was 10.4 atm prior to the reaction, what would be the expected pressure after the reaction was completed?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
Suppose the reaction between nitrogen and hydrogen was run according to the amounts presented in par...
Questions

Mathematics, 07.12.2020 06:00

Biology, 07.12.2020 06:00

Mathematics, 07.12.2020 06:00

History, 07.12.2020 06:00


Mathematics, 07.12.2020 06:00




History, 07.12.2020 06:00




Computers and Technology, 07.12.2020 06:00

Health, 07.12.2020 06:00





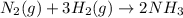
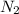 + moles of
+ moles of 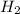 = 0.908 mol
= 0.908 mol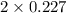 = 0.454 mol
= 0.454 mol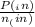 =
= 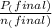
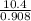 =
= 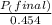
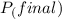 =
=