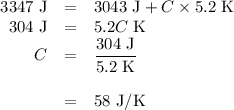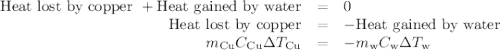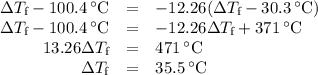Chemistry, 18.10.2019 17:10 tonimgreen17p6vqjq
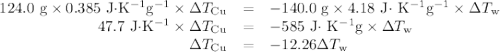
Acoffee-cup calorimeter contains 140.0 g of water at 25.1°c . a 124.0-g block of copper metal is heated to 100.4°c by putting it in a beaker of boiling water. the specific heat of cu(s) is 0.385 j/g⋅k. the cu is added to the calorimeter, and after a time the contents of the cup reach a constant temperature of 30.3°c .
(a)- determine the amount of heat, in j, lost by the copper block. enter the absolute amount of heat lost by cu, without a minus sign.
(b)- determine the amount of heat gained by the water. the specific heat of water is 4.18 j/g⋅k.
(c)-the difference between your answers for (a) and (b) is due to heat loss through the styrofoam → cups and the heat necessary to raise the temperature of the inner wall of the apparatus. the heat
capacity of the calorimeter is the amount of heat necessary to raise the temperature of the apparatus (the cups and the stopper) by 1 k. calculate the heat capacity of the calorimeter in j/k.
expressyour answer using two significant figures.
(d)-what would be the final temperature of the system if all the heat lost by the copper block were absorbed by the water in the calorimeter?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2



Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
Acoffee-cup calorimeter contains 140.0 g of water at 25.1°c . a 124.0-g block of copper metal is hea...
Questions

History, 25.10.2019 17:43

English, 25.10.2019 17:43



Geography, 25.10.2019 17:43


Chemistry, 25.10.2019 17:43

Mathematics, 25.10.2019 17:43



Business, 25.10.2019 17:43





Biology, 25.10.2019 17:43

Spanish, 25.10.2019 17:43

Mathematics, 25.10.2019 17:43

English, 25.10.2019 17:43