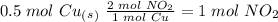Chemistry, 18.10.2019 03:30 GamerGirl15
4. copper metal reacts with nitric acid(hno3) to produce aqueous copper (ii)nitrate, nitrogen dioxide gas and liquid water. a. write the balanced equation for the reaction b. if there are 0.500 moles of copper metal present how many moles of nitric acid are required for the reaction? how many moles of nitrogen dioxide gas are formed?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 11:30
Jenny places a strip of ph paper into a solution. when she removes the ph paper, it has turned yellow-green. what should jenny do next to determine the ph of her solution? a. use a different testing method because the ph paper should not change colors b. place the ph paper into a machine that reads the ph of the solution c. compare the ph paper's color with the color of ph paper from another solution d. compare the ph paper's color with a chart of colors and ph ranges
Answers: 1
You know the right answer?
4. copper metal reacts with nitric acid(hno3) to produce aqueous copper (ii)nitrate, nitrogen dioxid...
Questions












Biology, 19.11.2019 01:31








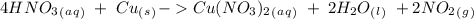
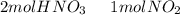
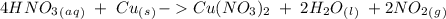
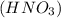 we have to use the molar ratio in the balence reaction:
we have to use the molar ratio in the balence reaction: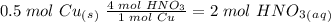
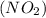 we have to follow the same logic:
we have to follow the same logic: