
Chemistry, 11.10.2019 22:30 adhanom1271
Magnesium (used in the manufacture of light alloys) reacts with iron(iii) chloride to form magnesium chloride and iron. 3mg(s) + 2fecl₃(s) → 3mgcl₂(s) + 2fe(s) a mixture of 41.0 g of magnesium ( = 24.31 g/mol) and 175 g of iron(iii) chloride ( = 162.2 g/mol) is allowed to react. identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:50
How do the energy differences between the higher energy levels of an atom compare with the energy difference between the lower energy level of the atom
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

You know the right answer?
Magnesium (used in the manufacture of light alloys) reacts with iron(iii) chloride to form magnesium...
Questions










English, 07.05.2020 07:58



Mathematics, 07.05.2020 07:58

Chemistry, 07.05.2020 07:58

Arts, 07.05.2020 07:58



Mathematics, 07.05.2020 07:58

Computers and Technology, 07.05.2020 07:58

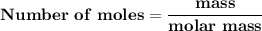
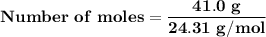
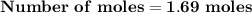
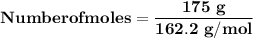
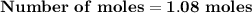
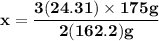 x = 39.34 grams
x = 39.34 grams

