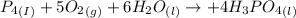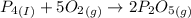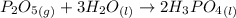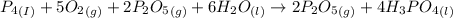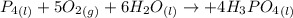Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for dental and orthopedic use, can be synthesized using a two-step thermal process. in the first step, phosphorus and oxygen react to form diphosphorus pentoxide: (l)(g)(g) in the second step, diphosphorus pentoxide and water react to form phosphoric acid: (g)(l)(l) write the net chemical equation for the production of phosphoric acid from phosphorus, oxygen and water. be sure your equation is balanced.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a concentration of 5.2 × 10–8 m h3o+?
Answers: 1

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Phosphoric acid, which is commonly used as rust inhibitor, food additive and etching agent for denta...
Questions


Mathematics, 15.07.2019 16:30


History, 15.07.2019 16:30

History, 15.07.2019 16:30

Mathematics, 15.07.2019 16:30


Mathematics, 15.07.2019 16:30



Health, 15.07.2019 16:30

Geography, 15.07.2019 16:30




History, 15.07.2019 16:30




Mathematics, 15.07.2019 16:30