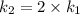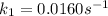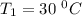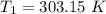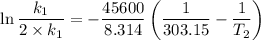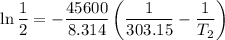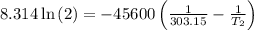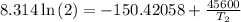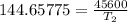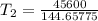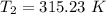Chemistry, 08.10.2019 03:20 jonlandis6
The arrhenius equation shows the relationship between the rate constant k and the temperature t in kelvins and is typically written as k=ae−ea/rt where r is the gas constant (8.314 j/mol⋅k), a is a constant called the frequency factor, and ea is the activation energy for the reaction. however, a more practical form of this equation is lnk2k1=ear(1t1−1t2) which is mathematically equivalent to lnk1k2=ear(1t2−1t1) where k1 and k2 are the rate constants for a single reaction at two different absolute temperatures (t1 and t2). part a the activation energy of a certain reaction is 45.6 kj/mol . at 30 ∘c , the rate constant is 0.0160s−1 . at what temperature in degrees celsius would this reaction go twice as fast

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
You know the right answer?
The arrhenius equation shows the relationship between the rate constant k and the temperature t in k...
Questions

Mathematics, 03.02.2021 05:00

English, 03.02.2021 05:00

Mathematics, 03.02.2021 05:00

Mathematics, 03.02.2021 05:00

History, 03.02.2021 05:00


Mathematics, 03.02.2021 05:00

Mathematics, 03.02.2021 05:00

Mathematics, 03.02.2021 05:00

Mathematics, 03.02.2021 05:00

Mathematics, 03.02.2021 05:00


Mathematics, 03.02.2021 05:00

Mathematics, 03.02.2021 05:00

Mathematics, 03.02.2021 05:00

English, 03.02.2021 05:00

Mathematics, 03.02.2021 05:00

Mathematics, 03.02.2021 05:00


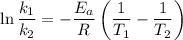
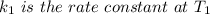
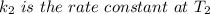
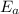 is the activation energy
is the activation energy