
Chemistry, 01.10.2019 21:10 ofcitsnijah
Kcl and kbr are both ionic solids. a mixture of kcl and kbr has a mass of 3.595 g. when this mixture is heated in the presence of excess cl2, all of the kbr is converted to kcl. if the total mass of kcl present after this reaction is 3.129 g, what percentage (by mass) of the original mixture was kbr? (hint: be sure that you understand why the mass of the sample has decreased. it may if you write an equation for the reaction that converted the kbr to kcl.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1

Chemistry, 23.06.2019 08:30
Benzonitrile (c6h5cn) is reduced to two different products depending on the reducing agent used. treatment with lithium aluminum hydride followed by water forms k, which has a molecular ion in its mass spectrum at 107 and the following ir absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. treatment with a milder reducing agent forms l, which has a molecular ion in its mass spectrum at 106 and the following ir absorptions: 3086, 2850, 2820, 2736, 1703, and 1600 cm-1. l shows fragments in its mass spectrum at m/z = 105 and 77. propose structures for k and l and choose an explanation for how this could be concluded.
Answers: 3
You know the right answer?
Kcl and kbr are both ionic solids. a mixture of kcl and kbr has a mass of 3.595 g. when this mixture...
Questions


















Mathematics, 23.10.2019 01:30


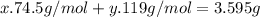
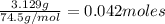
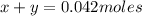
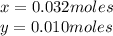
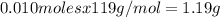
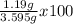 %
% 

