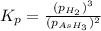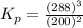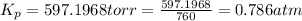Chemistry, 01.10.2019 04:20 gadgetady5699
The gas arsine (ash3) decomposes as follows: 2ash3(g) < > 2as(s) + 3h2(g) in an experiment pure ash3(g) was placed in an empty, rigid, sealed flask at a pressure of 392.0 torr. after 48 h the pressure in the flask was observed to be constant at 488.0 torr. a. calculate the equilibrium pressure of h2(g). b. calculate kp for this reaction.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2


Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
The gas arsine (ash3) decomposes as follows: 2ash3(g) < > 2as(s) + 3h2(g) in an experiment p...
Questions

History, 21.10.2020 01:01


Mathematics, 21.10.2020 01:01

Biology, 21.10.2020 01:01

Computers and Technology, 21.10.2020 01:01


Mathematics, 21.10.2020 01:01

History, 21.10.2020 01:01



Mathematics, 21.10.2020 01:01



Mathematics, 21.10.2020 01:01

Social Studies, 21.10.2020 01:01



Social Studies, 21.10.2020 01:01


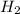 gas is, 288 torr
gas is, 288 torr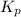 for this reaction is, 0.786 atm
for this reaction is, 0.786 atm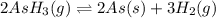
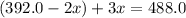
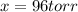
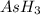 at equilibrium = (392.0-2x) = [392.0-2(96)] = 200 torr
at equilibrium = (392.0-2x) = [392.0-2(96)] = 200 torr