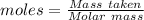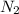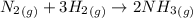Ammonia is produced by the reaction of nitrogen and hydrogen according to the equation n2(g) + 3h2(g) → 2nh3(g) calculate the mass of ammonia produced when 37.0 g of nitrogen react with 12.0 g of hydrogen. g nh3 which is the excess reactant and how much of it will be left over when the reaction is complete? hydrogen nitrogen

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1


Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
Ammonia is produced by the reaction of nitrogen and hydrogen according to the equation n2(g) + 3h2(g...
Questions


Social Studies, 30.11.2019 11:31

English, 30.11.2019 11:31

History, 30.11.2019 11:31



Mathematics, 30.11.2019 11:31

Social Studies, 30.11.2019 11:31


History, 30.11.2019 11:31


English, 30.11.2019 11:31



Mathematics, 30.11.2019 11:31

Mathematics, 30.11.2019 11:31



Computers and Technology, 30.11.2019 11:31

Chemistry, 30.11.2019 11:31

 .
.