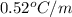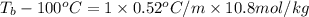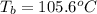Chemistry, 27.09.2019 00:30 Seaqueen3103
How many liters of the antifreeze ethylene glycol [ch2(oh)ch2(oh)] would you add to a car radiator containing 6.50 l of water if the coldest winter temperature in your area is –20ºc? calculate the boiling point of this water-ethylene glycol mixture. (te density of ethylene glycol is 1.11 g/ml.) kf = 1.86 ºc /m

Answers: 2
Another question on Chemistry



Chemistry, 23.06.2019 06:00
Nthis lab, you will do experiments to identify types of changes. using the question format you learned (shown above), write an investigative question that you can answer by doing these experiments
Answers: 3

You know the right answer?
How many liters of the antifreeze ethylene glycol [ch2(oh)ch2(oh)] would you add to a car radiator c...
Questions

History, 30.01.2021 22:00


Physics, 30.01.2021 22:00




Computers and Technology, 30.01.2021 22:00

Computers and Technology, 30.01.2021 22:00



Health, 30.01.2021 22:00



Mathematics, 30.01.2021 22:00

Mathematics, 30.01.2021 22:00



Mathematics, 30.01.2021 22:00

Health, 30.01.2021 22:00

Advanced Placement (AP), 30.01.2021 22:00

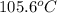
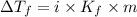
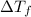 = change in freezing point =
= change in freezing point = 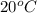
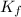 = freezing point constant =
= freezing point constant = 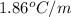
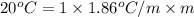
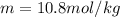
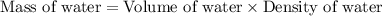
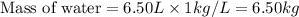
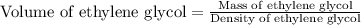
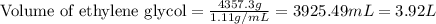
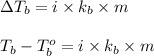
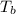 = boiling point of solution = ?
= boiling point of solution = ?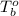 = boiling point of pure water =
= boiling point of pure water = 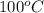
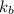 = boiling point constant =
= boiling point constant =