
Chemistry, 22.09.2019 05:30 rosehayden21
The n2o4−no2 reversible reaction is found to have the following equilibrium partial pressures at 100∘c. calculate kp for the reaction. n2o4(g)⇌2no2(g) 0.0005 atm 0.095 atm express your answer using two significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2


Chemistry, 23.06.2019 17:30
Alithium atom has three protons, three neutrons, and three electrons. what is the overall charge on this atom?
Answers: 1
You know the right answer?
The n2o4−no2 reversible reaction is found to have the following equilibrium partial pressures at 100...
Questions



Mathematics, 03.02.2021 20:00

Chemistry, 03.02.2021 20:00






Mathematics, 03.02.2021 20:00



English, 03.02.2021 20:00


Mathematics, 03.02.2021 20:00

Mathematics, 03.02.2021 20:00

History, 03.02.2021 20:00


Mathematics, 03.02.2021 20:00

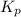 for the reaction is 18.05
for the reaction is 18.05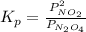
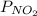 and
and 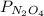 are equilibrium partial pressure of
are equilibrium partial pressure of 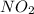 and
and 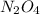 respectively
respectively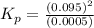 = 18.05
= 18.05


