

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Which of the following would have the largest amount of mass?
1 nanogram (ng)
1 kilogra...
1 nanogram (ng)
1 kilogra...
Questions

Mathematics, 26.02.2021 03:00

English, 26.02.2021 03:00


History, 26.02.2021 03:00




Mathematics, 26.02.2021 03:00


Mathematics, 26.02.2021 03:00

Chemistry, 26.02.2021 03:00

Mathematics, 26.02.2021 03:00


Mathematics, 26.02.2021 03:00

Mathematics, 26.02.2021 03:00





Mathematics, 26.02.2021 03:00

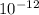 kilograms, a milligram is equivalent to
kilograms, a milligram is equivalent to 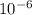 kilograms and a centigram is equivalent to
kilograms and a centigram is equivalent to 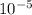 kilograms, which means all nanograms, milligrams, and centigrams are smaller units than kilograms.
kilograms, which means all nanograms, milligrams, and centigrams are smaller units than kilograms.

