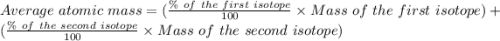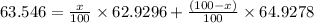Chemistry, 19.09.2019 16:30 xxaurorabluexx
Copper has two naturally occurring isotopes, 63cu (isotopic mass 62.9296 amu) and 65cu (isotopic mass 64.9278 amu). if copper has an atomic mass of 63.546 amu, what is the percent abundance of each isotope? report your answer to 5 significant figures.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1
You know the right answer?
Copper has two naturally occurring isotopes, 63cu (isotopic mass 62.9296 amu) and 65cu (isotopic mas...
Questions

English, 17.11.2020 22:30



Mathematics, 17.11.2020 22:30

History, 17.11.2020 22:30

Mathematics, 17.11.2020 22:30



Mathematics, 17.11.2020 22:30


Mathematics, 17.11.2020 22:30

Mathematics, 17.11.2020 22:30


Mathematics, 17.11.2020 22:30


English, 17.11.2020 22:30



Business, 17.11.2020 22:30

Biology, 17.11.2020 22:30