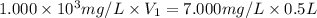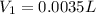Chemistry, 19.09.2019 01:00 student0724
If a chemist wishes to dilute a 1.000 × 10^3 mg/l stock solution to prepare 5.000 × 10^2 ml of a working standard that has a concentration of 7.000 mg/l, what volume of the 1.000 × 10^3 mg/l standard solution is needed?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1


Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 11:30
Bridget is in science class. her teacher gives her two unknown substances and asks her to determine their relative ph. she places a piece of red litmus paper into both substances. the litmus paper turns purple when she places it into substance i. the litmus paper turns blue when she places it into substance ii. a. substance i is a neutral substance and substance ii is an acid. b. substance i is a neutral substance and substance ii is a base. c. substance i is an acid and substance ii is a base. d. substance i is a base and substance ii is a neutral substance.
Answers: 1
You know the right answer?
If a chemist wishes to dilute a 1.000 × 10^3 mg/l stock solution to prepare 5.000 × 10^2 ml of a wor...
Questions




Mathematics, 23.11.2020 01:50



Health, 23.11.2020 01:50

Mathematics, 23.11.2020 01:50

Chemistry, 23.11.2020 01:50


Mathematics, 23.11.2020 01:50

Mathematics, 23.11.2020 01:50

Computers and Technology, 23.11.2020 01:50

Mathematics, 23.11.2020 01:50



Mathematics, 23.11.2020 01:50

History, 23.11.2020 01:50

Mathematics, 23.11.2020 01:50

Biology, 23.11.2020 01:50


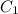 = molarity of stock solution =
= molarity of stock solution = 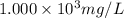
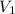 = volume of stock solution = ?
= volume of stock solution = ?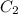 = molarity of diluted solution =
= molarity of diluted solution = 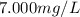
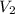 = volume of diluted solution =
= volume of diluted solution = 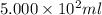 = 0.5 L (1L=1000 ml)
= 0.5 L (1L=1000 ml)