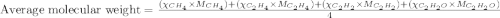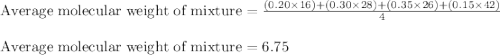Chemistry, 13.09.2019 22:30 samanthahurtado0914
Agaseous mixture composed of 20% ch4, 30% c2h4, 35% c2h2, and 15% c2h20. what is the average molecular weight of the mixture? a) 20 b) 9.25 c) 25 d) 6.75

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
Agaseous mixture composed of 20% ch4, 30% c2h4, 35% c2h2, and 15% c2h20. what is the average molecul...
Questions

History, 23.06.2019 20:00

History, 23.06.2019 20:00

History, 23.06.2019 20:00


Social Studies, 23.06.2019 20:00

Social Studies, 23.06.2019 20:00

English, 23.06.2019 20:00

Mathematics, 23.06.2019 20:00

Mathematics, 23.06.2019 20:00


Mathematics, 23.06.2019 20:00

Mathematics, 23.06.2019 20:00


Mathematics, 23.06.2019 20:00

Mathematics, 23.06.2019 20:00




Mathematics, 23.06.2019 20:00

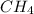 = 20 %
= 20 %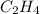 = 30 %
= 30 %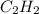 = 35 %
= 35 %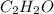 = 15 %
= 15 %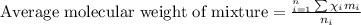
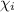 = mole fractions of i-th species
= mole fractions of i-th species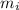 = molar masses of i-th species
= molar masses of i-th species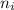 = number of observations
= number of observations