

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
2.4 bromium has two naturally occurring isotopes: 79br, with an atomic weight of 78.918 amu, and 81...
Questions

Biology, 29.12.2019 07:31


English, 29.12.2019 07:31




History, 29.12.2019 07:31






Mathematics, 29.12.2019 07:31

English, 29.12.2019 07:31

Mathematics, 29.12.2019 07:31

Mathematics, 29.12.2019 07:31


History, 29.12.2019 07:31


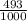 81Br and
81Br and 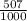 79BR
79BR

