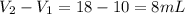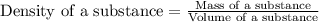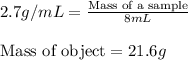Chemistry, 10.09.2019 13:10 Student3220
Asample of aluminum is placed in a 25 ml graduated cylinder containing 10 ml of water. the level of water rises to 18 ml. aluminum has a density of 2.7 g/ml. calculate the mass of the sample
include explanation if possible

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
Asample of aluminum is placed in a 25 ml graduated cylinder containing 10 ml of water. the level of...
Questions



Physics, 22.09.2021 14:00



Mathematics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00





Mathematics, 22.09.2021 14:00


English, 22.09.2021 14:00



Mathematics, 22.09.2021 14:00

Mathematics, 22.09.2021 14:00

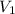 = 10 mL
= 10 mL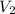 = 18 mL
= 18 mL