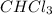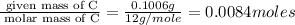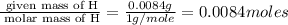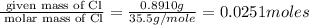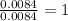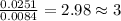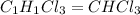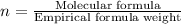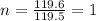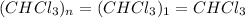Chemistry, 10.09.2019 01:10 gomezjuana123
Acompound was found to contain 10.06% carbon, 89.10% chlorine, and 0.84% hydrogen, by mass. if the molar mass of the compound was found to be 119.6 g/mol, its molecular formula will be

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
Acompound was found to contain 10.06% carbon, 89.10% chlorine, and 0.84% hydrogen, by mass. if the m...
Questions





Mathematics, 18.03.2021 02:40



Mathematics, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40


English, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40


Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40