
Chemistry, 09.09.2019 19:30 reaperdraws
Which of the following solutes will lower the freezing point of water the most?
a.
the molecular compound sucrose (c12h22o11)
b.
the ionic compound magnesium sulfate (mgso4)
c.
the ionic compound lithium chloride (licl)
d.
the ionic compound calcium fluoride (caf2)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Which of the following solutes will lower the freezing point of water the most?
a.
the...
a.
the...
Questions


Social Studies, 30.09.2019 22:00

Business, 30.09.2019 22:00

Biology, 30.09.2019 22:00

Social Studies, 30.09.2019 22:00

History, 30.09.2019 22:00

History, 30.09.2019 22:00

Chemistry, 30.09.2019 22:00




English, 30.09.2019 22:00

History, 30.09.2019 22:00



Business, 30.09.2019 22:00

Mathematics, 30.09.2019 22:00


Health, 30.09.2019 22:00

English, 30.09.2019 22:00

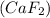
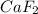 and the freezing point will be lowest.
and the freezing point will be lowest.

