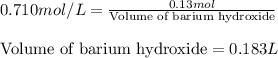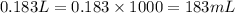Chemistry, 06.09.2019 22:10 zmoore8015
Calculate the number of milliliters of 0.710 m ba(oh)2 required to precipitate all of the mn2+ ions in 161 ml of 0.796 m kmno4 solution as mn(oh)2. the equation for the reaction is: mnso4(aq) + ba(oh)2(aq) mn(oh)2(s) + baso4(aq)

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 00:30
Many bird species build nests in which they raise their offspring. one of these species is known as the magnolia warbler. breeding pairs of magnolia warblers build nests out of pieces of grass and twigs. the female warbler will then lay her eggs in the nest and sit on the eggs for a few weeks until they hatch. after the offspring have hatched, the female will continue to sit on the newborn chicks to keep them warm. both the male and the female bring food for the offspring to eat until the young are mature enough to find food on their own. which of these most likely describes why birds such as the magnolia warbler build nests in which to raise their offspring? a. nest building decreases the amount of energy used by the parent birds to raise their offspring to adulthood. b. nest building increases the probability that the offspring will survive and eventually produce offspring of their own. c. nest building decreases the amount of food that the offspring require the adult birds to provide. d. nest building increases the probability of the offspring being located by airborne predators.
Answers: 1
You know the right answer?
Calculate the number of milliliters of 0.710 m ba(oh)2 required to precipitate all of the mn2+ ions...
Questions


Social Studies, 06.11.2019 06:31






Chemistry, 06.11.2019 06:31




Mathematics, 06.11.2019 06:31

Mathematics, 06.11.2019 06:31


Biology, 06.11.2019 06:31



Mathematics, 06.11.2019 06:31


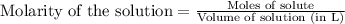 .....(1)
.....(1)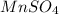 = 0.796 M
= 0.796 M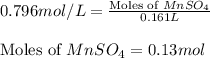
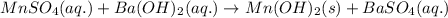
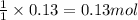 of barium hydroxide
of barium hydroxide