Write half-reactions for the oxidation and reduction process for each of the following.
a. fe...

Chemistry, 05.09.2019 20:30 hamadehassan
Write half-reactions for the oxidation and reduction process for each of the following.
a. fe2+ + mno4 - fe3+ + mn2+
b. sn2+ + io3 - sn4+ + i-
c. s2- + no3 - s + no
d. nh3 + no2 n2 + h2o

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
You know the right answer?
Questions


Mathematics, 28.05.2021 01:00


Computers and Technology, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00


Spanish, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00

Mathematics, 28.05.2021 01:00





Mathematics, 28.05.2021 01:00

Computers and Technology, 28.05.2021 01:00

Arts, 28.05.2021 01:00


Biology, 28.05.2021 01:00


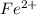 ⇒
⇒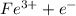
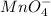 + 2
+ 2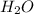 + 3
+ 3 ⇒
⇒ 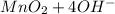
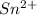 ⇒
⇒ 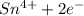
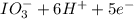 ⇒
⇒ 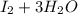
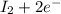 ⇒
⇒
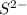 ⇒
⇒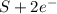
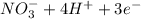 ⇒
⇒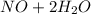
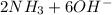 ⇒
⇒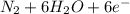
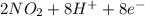 ⇒
⇒


