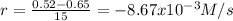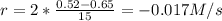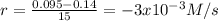a + b --> 2c

Chemistry, 30.08.2019 23:30 angsoccer02
Concentration time data were collected for the following reaction at 30 oc
a + b --> 2c
concentration time data at t = 30oc
time (s) [a] (mol l-1)
0 0.65
15 0.52
30 0.35
45 0.28
60 0.22
75 0.14
90 0.095
(i) calculate the average rate of consumption of a in the first 15 seconds of reaction.
(ii) calculate the average rate of production of c in the first 15 seconds of reaction.
(iii) calculate the average rate of consumption of a in the last 15 seconds of the reaction.
(iv) explain the difference between the rates of consumption calculated in (i) and that in (iii)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
Concentration time data were collected for the following reaction at 30 oc
a + b --> 2c
a + b --> 2c
Questions

Mathematics, 27.09.2020 14:01


Mathematics, 27.09.2020 14:01

Mathematics, 27.09.2020 14:01

Mathematics, 27.09.2020 14:01

Mathematics, 27.09.2020 14:01




Mathematics, 27.09.2020 15:01

English, 27.09.2020 15:01


Social Studies, 27.09.2020 15:01


Mathematics, 27.09.2020 15:01



Mathematics, 27.09.2020 15:01

Mathematics, 27.09.2020 15:01

Social Studies, 27.09.2020 15:01

![r=\frac{\Delta [Concentration]}{\Delta t}](/tpl/images/0213/3224/c01cf.png)