Chemistry, 27.08.2019 16:20 prettygirlgwen24
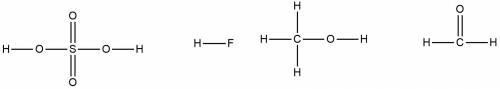
Which of the following molecules is/are expected to form hydrogen bonds in the liquid state or solid state: h2so4, hf, ch3oh, ch2o (formaldehyde)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 21.06.2019 17:10
The concept of empiricism states that all rationally accepted knowledge is determined from experience. francis bacon was one of the first scientists to promote this theory. what was it’s impact on society?
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
Which of the following molecules is/are expected to form hydrogen bonds in the liquid state or solid...
Questions




Chemistry, 21.06.2019 21:00

Mathematics, 21.06.2019 21:00


Mathematics, 21.06.2019 21:00

Computers and Technology, 21.06.2019 21:00



Mathematics, 21.06.2019 21:00



Advanced Placement (AP), 21.06.2019 21:00






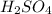 , HF and
, HF and 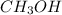 are expected to form hydrogen bonds in the liquid or solid state.
are expected to form hydrogen bonds in the liquid or solid state.