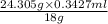The solubility of water in diethyl ether has been reported to be 1.468 % by mass.' assuming that 23.0 ml of diethyl ether were allowed to become saturated with water before used and that the 1.2 g magnesium was the limiting reagent, what percentage of the product is expected to be lost due to the water in the solvent if the ether is not anhydrous?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
The solubility of water in diethyl ether has been reported to be 1.468 % by mass.' assuming that 23....
Questions

History, 28.03.2020 00:56

Health, 28.03.2020 00:56

Mathematics, 28.03.2020 00:56






Chemistry, 28.03.2020 00:56

Mathematics, 28.03.2020 00:56

English, 28.03.2020 00:56

Mathematics, 28.03.2020 00:56


Engineering, 28.03.2020 00:56





Mathematics, 28.03.2020 00:56

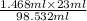
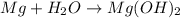
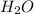 = 18 g
= 18 g