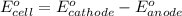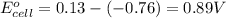Chemistry, 20.08.2019 05:10 strongl3219
Agalvanic (voltaic) cell consists of an electrode composed of zinc in a 1.0 m zinc ion solution and another electrode composed of copper in a 1.0 m copper(ii) ion solution, connected by a salt bridge. calculate the standard potential for this cell at 25 °c. standard reduction potentials can be found here.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

You know the right answer?
Agalvanic (voltaic) cell consists of an electrode composed of zinc in a 1.0 m zinc ion solution and...
Questions




Mathematics, 11.10.2019 11:00

Social Studies, 11.10.2019 11:00

Social Studies, 11.10.2019 11:00

English, 11.10.2019 11:00






Mathematics, 11.10.2019 11:00



Health, 11.10.2019 11:00


English, 11.10.2019 11:00


Health, 11.10.2019 11:00

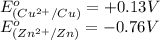
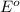 potential will always get reduced and will undergo reduction reaction. Here, copper will undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, copper will undergo reduction reaction will get reduced.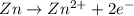
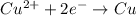
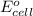 of the reaction, we use the equation:
of the reaction, we use the equation: