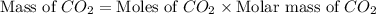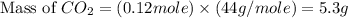Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 1.74 g of butane is mixed with 11. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 23.06.2019 05:50
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions

Mathematics, 21.09.2020 14:01



English, 21.09.2020 14:01



History, 21.09.2020 14:01

Mathematics, 21.09.2020 14:01

Mathematics, 21.09.2020 14:01

English, 21.09.2020 14:01




Mathematics, 21.09.2020 14:01




Mathematics, 21.09.2020 14:01

Mathematics, 21.09.2020 14:01

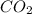 produced will be, 5.3 grams.
produced will be, 5.3 grams.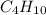 = 1.74 g
= 1.74 g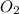 = 11 g
= 11 g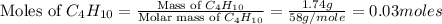
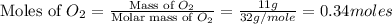
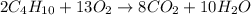
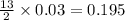 moles of
moles of 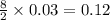 moles of
moles of