
Chemistry, 08.08.2019 06:20 babygirllll2071
Astudent weighs out 8.21 g of (nh4)2s, transfers it to a 500. ml volumetric flask, adds enough water to dissolve the solid and then adds water to the 500 ml mark on the neck of the flask. calculate the concentration (in molarity units) of ammonium sulfide in the resulting solution?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 23.06.2019 16:50
How can a scientist assess whether a pure niobium (nb) sample is responsible for contaminating the lab with radioactivity? test the niobium sample to see whether it now contains other elements.test the niobium sample for the presence of niobium oxide compounds.heat the niobium, and see if the level of radioactivity in the lab increases.place the niobium under pressure, and see if the level of radioactivity in the lab increases.
Answers: 3
You know the right answer?
Astudent weighs out 8.21 g of (nh4)2s, transfers it to a 500. ml volumetric flask, adds enough water...
Questions

French, 31.07.2019 01:30

Computers and Technology, 31.07.2019 01:30


English, 31.07.2019 01:30



English, 31.07.2019 01:30


History, 31.07.2019 01:30



English, 31.07.2019 01:30

Mathematics, 31.07.2019 01:30

Computers and Technology, 31.07.2019 01:40

Computers and Technology, 31.07.2019 01:40


Biology, 31.07.2019 01:40



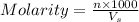
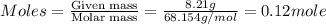
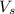 = volume of solution in ml = 500 ml
= volume of solution in ml = 500 ml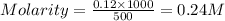
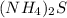 is 0.24 M.
is 0.24 M.

