Chemistry, 06.08.2019 05:10 palomaresmitchelle
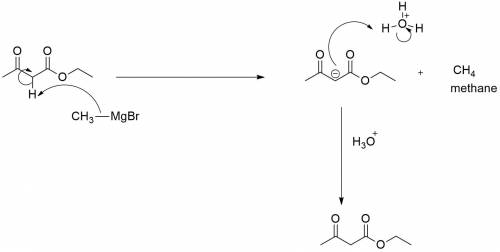
When ethyl acetoacetate (ch3coch2co2ch2ch3) is treated with one equivalent of ch3mgbr, a gas is evolved from the reaction mixture, and after adding aqueous acid, ethyl acetoacetate is recovered in high yield. identify the gas formed and explain why the starting material was recovered in this reaction. be sure to answer all parts.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
When ethyl acetoacetate (ch3coch2co2ch2ch3) is treated with one equivalent of ch3mgbr, a gas is evol...
Questions

Mathematics, 21.09.2019 18:10



Mathematics, 21.09.2019 18:10


Physics, 21.09.2019 18:10



Computers and Technology, 21.09.2019 18:10









Social Studies, 21.09.2019 18:10


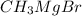 acts as base towards ethyl acetoacetate. Because ethyl acetoacetate contains active methylene group.
acts as base towards ethyl acetoacetate. Because ethyl acetoacetate contains active methylene group.